Abstract
Introduction: Recombinant adeno-associated virus (rAAV) vectors have shown encouraging results as candidates for in vivo gene therapy across several indications (eg, cystic fibrosis, hemophilia). However, pre-existing neutralizing antibodies (NAbs) against AAV serotypes can interfere with effective target cell transduction by rAAV vectors. Although there are limited data on the prevalence of NAbs, previous studies have described geographic variability. Therefore, we performed a multi-country, observational, retrospective, cross-sectional study to assess the prevalence and titer levels of 9 serotypes of NAbs in adults (age ≥16 years) and children (age ˂16 years) and by various subgroups (eg, geographic region, biological sex, ethnicity). This summary includes results for 6 serotypes (AAV1, AAV5, AAV6, AAV8, AAV9, and AAV-Spark100).
Methods: Participants from 10 countries (Australia, Canada, France, Germany, Italy, Japan, South Korea, Spain, the UK, and US) were enrolled from previous non-gene therapy clinical studies conducted between 2015 and 2019. Serum samples from the study participants were obtained from the Pfizer Biobank, with data on demographics and clinical characteristics obtained from the clinical trial database. Having optimized the assay, serum samples were diluted (1:1, 1:2, 1:4, 1:8, 1:16, 1:80, 1:400, 1:2000, 1:10,000, 1:50,000) and analyzed by a central laboratory to determine the NAb titer. Continuous variables (eg, NAb titers) were summarized descriptively using mean, standard deviation, median, interquartile range, minimum, and maximum. Categorical variables (eg, clinical characteristics) were summarized descriptively using frequency tables.
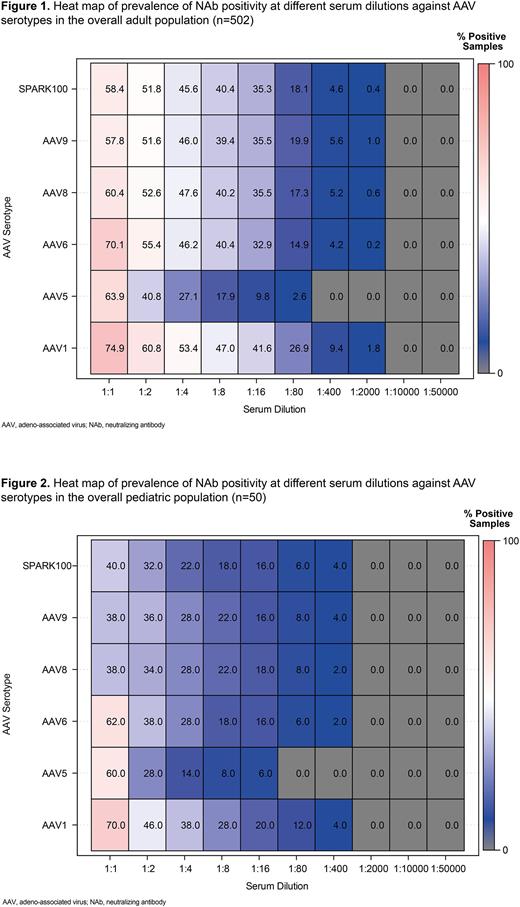
Results: A total of 552 samples were analyzed (59% were male; ages ˂16 years, n=50; 16-40 years, n=95; and 41-60 years, n=407), including 81 participants from the US. The primary analysis of the overall prevalence of NAb positivity (seroprevalence) in adults age ≥16 years (ie, proportion with detectable NAbs in serum samples) showed that the most prevalent NAb at a dilution of 1:1 was AAV1 (74.9%), followed by AAV6 (70.1%) and AAV5 (63.9%; Figure 1). At 1:2 and subsequent dilutions, AAV5 had the lowest seroprevalence among the AAV serotypes. Similar to adults, the primary analysis in children revealed that NAbs against AAV1 were the most prevalent and NAbs against AAV5 (starting at 1:2 dilution) were the least prevalent (Figure 2). Overall, samples in children exhibited lower seroprevalence compared with samples in adults, consistent with environmental exposure to natural infections during or following childhood. Co-prevalence of NAb positivity (adults and children combined) was most frequently observed for AAV1 and AAV6 across serum dilution levels, whereas co-prevalence of AAV5 with the other AAVs were less likely to be observed as the serum dilution level increased (eg, at 1:4 dilution, AAV1/AAV6 co-prevalence, 44.2%; AAV1/AAV5 co-prevalence 25.5%; AAV5/AAV6 co-prevalence 25.2%). The seroprevalence in adults varied by country. South Korea had the highest NAb prevalence against all of the AAV serotypes; samples from Japan, Australia, and the US exhibited the lowest seroprevalence. The data for adult participants suggested an association between age and NAb prevalence against all AAV serotypes, between biological sex and NAb prevalence against AAV9, and ethnicity and NAb prevalence against most AAV serotypes. The prevalence of NAbs in adults was observed to be higher in females, Asians, and older individuals. Within the US, there was a lower seroprevalence in the Midwest region versus the Northeast, South and West regions and, when dividing the US into 2 regions, there was a trend towards a higher seroprevalence in the southern versus the northern region. In general, exploratory findings suggest variation in the observed prevalence of NAbs against AAV serotypes by biological sex, ethnicity, age, and clinical disease area.
Conclusions: This large, global study of adult and pediatric participants from 10 countries provides new insights into the prevalence of NAbs against a range of clinically relevant AAV serotypes. Our results highlight variations in subgroups by geographic region, biological sex, ethnicity, age, and clinical disease.
Disclosures
Rasko:Bluebird Bio: Consultancy, Honoraria, Other: Supply of Material (MTA); Cynata: Consultancy, Honoraria, Other: Supply of Material (MTA); Genea: Current equity holder in publicly-traded company; Gilead: Consultancy, Honoraria, Other: Supply of Material (MTA); Novartis: Consultancy, Honoraria, Other: Supply of Material (MTA); Pfizer Inc.: Consultancy, Honoraria, Other: Supply of Material (MTA); RareCyte: Consultancy, Current equity holder in private company, Honoraria, Other: Supply of Material (MTA); Roche: Consultancy, Honoraria, Other: Supply of Material (MTA); Spark Therapeutics: Consultancy, Honoraria, Other: Supply of Material (MTA). Bashirians:Pfizer Inc.: Current Employment, Current equity holder in private company, Current equity holder in publicly-traded company, Current holder of stock options in a privately-held company, Divested equity in a private or publicly-traded company in the past 24 months. Petropoulos:Labcorp: Current Employment, Current equity holder in publicly-traded company, Other: Current Corporate Officer. Wrin:Labcorp: Current Employment, Current equity holder in publicly-traded company. Paliwal:Pfizer Inc.: Current Employment, Current equity holder in publicly-traded company. Somanathan:Pfizer Inc.: Current Employment, Current equity holder in publicly-traded company. Casey:Pfizer Inc.: Current Employment, Current equity holder in publicly-traded company. da Fonseca Pereira:Pfizer: Current Employment, Current equity holder in publicly-traded company, Ended employment in the past 24 months; Cytiva: Current Employment, Current equity holder in publicly-traded company, Ended employment in the past 24 months, Other: (Spouse). Winburn:Pfizer: Current Employment, Current equity holder in publicly-traded company. Chhabra:Pfizer Inc.: Current Employment, Current equity holder in publicly-traded company.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal